Samples of Popular Diabetes Drug Contain Potential Carcinogen, F.D.A. Says
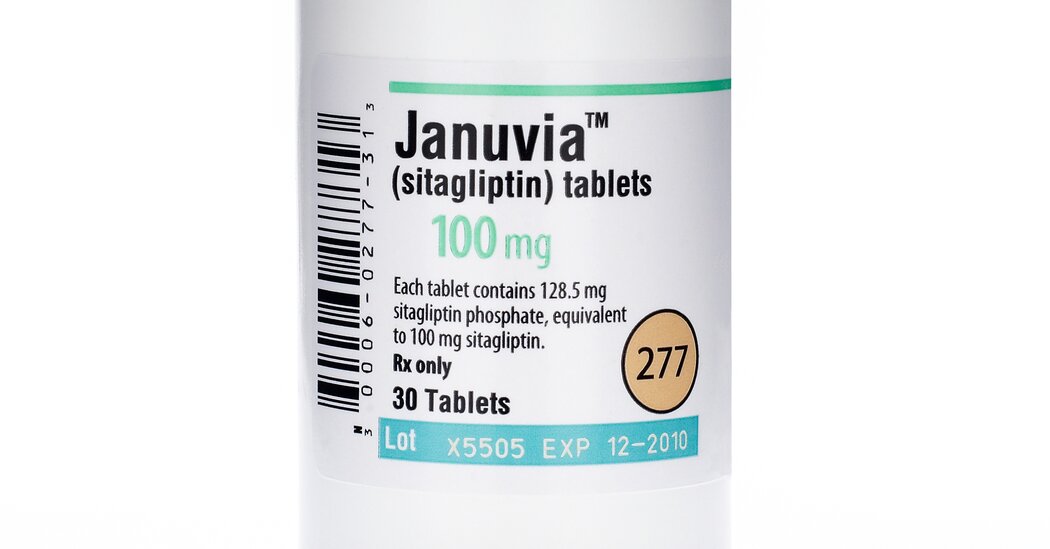
The Food and Drug Administration said that traces of a potential carcinogen had been found in samples of a popular diabetes drug produced by Merck, the latest instance in which impurities were detected in top-selling pharmaceutical products.
Millions of people with Type 2 diabetes rely on the drug, sitagliptin, to keep high blood sugar levels in check. Merck markets the drug as Januvia and Janumet. Last year, sitagliptin generated more than $5 billion in revenue for Merck and was its third best-selling product.
Despite finding the impurities in some batches, the F.D.A. will allow Merck to continue selling the drug temporarily, saying the risks are outweighed by the immediate medical needs of patients. “It could be dangerous for patients with this condition to stop taking their sitagliptin without first talking to their health care professional,” the agency said in a statement.
Merck, which first detected the contamination and reported it to regulators, said it was trying to address the problem and would work with health authorities around the world. “We remain confident in the safety, efficacy and quality of our sitagliptin-containing medicines,” the company said, adding that it did not anticipate shortages of the drug, which the F.D.A. first approved in 2006.
The impurity, known as NTTP, belongs to the family of nitrosamine compounds that have in recent years been discovered in a number of medications. Since 2018, federal regulators have alerted the public about nitrosamine contamination in samples of the heartburn medication Zantac, the antibiotic rifampin and the smoking-cessation drug Chantix.
The F.D.A. has described NTTP as a “probable human carcinogen,” based largely on laboratory testing. The agency lacks data to evaluate the carcinogenic potential of NTTP directly and instead said it used information about a closely related compound to determine exposure limits. Scientists at the agency have set a lifetime exposure to nitrosamine in medication at 37 nanogram per day, though it will allow up to 246 nanograms in sitagliptin for the time being.
In its statement, the F.D.A. called the additional cancer risk “minimal.”
