A Promising Trial Targets a Genetic Risk for Alzheimer’s
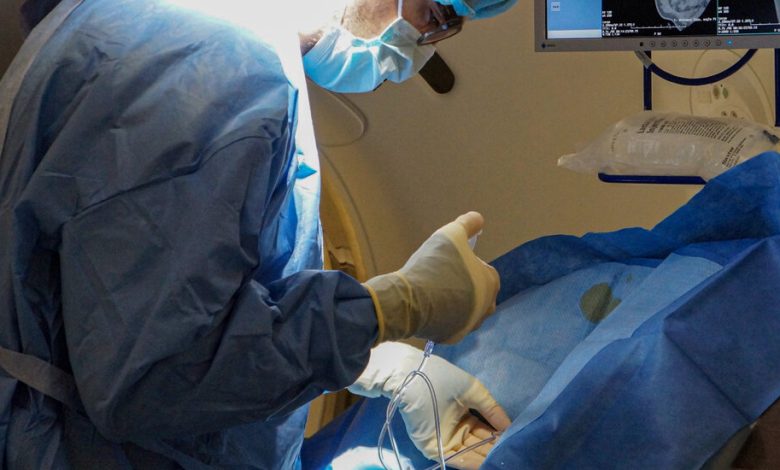
In a bold attempt to stop the progress of some cases of Alzheimer’s disease, a group of researchers is trying something new — injecting a protective gene into patients’ brains.
The trial involved just five patients with a particular genetic risk for Alzheimer’s. They received a very low dose of the gene therapy — a test of safety, which the treatment passed. But the preliminary results, announced Friday during the Clinical Trials on Alzheimer’s Disease conference, showed that proteins from the added gene appeared in the patients’ spinal fluid, and levels in the brain of two markers of Alzheimer’s disease, tau and amyloid, fell. Those findings were promising enough to advance the clinical trial into its next phase.
Treatment of another five patients at a higher dose is underway, and the work, initially funded by the nonprofit Alzheimer’s Drug Discovery Foundation, is supported by Lexeo Therapeutics, a fledgling company founded by Dr. Ronald Crystal, who is also chairman of the department of genetic medicine at Weill Cornell Medicine in New York. The hope is to get a stronger response, eventually leading to a treatment that might slow the disease in whom it has started or, even better, protect people at high risk who have no symptoms.
Experts not involved in the trial are fascinated.
“It’s a very provocative, very intriguing approach,” said Dr. Eliezer Masliah, director of the neuroscience division at the National Institute on Aging.
Participants in the study are among the approximately 2 percent of people who have inherited a pair of copies of a gene, APOE4, which markedly increases their risk of Alzheimer’s. For the study subjects, the first symptoms of Alzheimer’s had already emerged — their genetic risk had played out, and they had few options. There is no treatment that is directed specifically at APOE4-driven Alzheimer’s, nor is one on the near horizon.
“We’ve known about this risk factor for almost 30 years now,” said Dr. Howard Fillit, co-founder and chief scientific officer at the Alzheimer’s Drug Discovery Foundation. His foundation and other funders have supported efforts to fix the effects of APOE4 or to treat it with drugs, but to no avail.
With genetic tests like 23andMe readily available, more and more people are learning that they have two copies of APOE4. For some, like Chris Hemsworth, the 39-year-old star of “Thor,”, the knowledge is life-changing. In an unlikely coincidence, he got the genetic test as part of a documentary show he was making about life extension. When he learned the result, he decided to take a break from acting.
It is not clear exactly how APOE4 makes Alzheimer’s more likely or why some people with two copies of the variant never get the disease.
What is known is that APOE4 is one of three gene variants that affect the chances of a person having Alzheimer’s. The others are APOE3 and APOE2. Each person inherits two APOE gene variants, and the combination determines risk.
Compared to the most common variant, APOE3, having at least one copy of the APOE4 variant increases risk, and having an APOE2 variant decreases risk.
But estimating the lifetime risk conferred by these variants is tricky. The best data, said Dr. Deborah L. Blacker, a geriatric psychiatrist and epidemiologist at Massachusetts General Hospital, indicate that the lifetime risk of Alzheimer’s for those with two APOE4 genes is 30 percent to 55 percent. The lifetime risk in people with one APOE4 gene and one APOE2 gene has not been directly estimated but has appeared to be about 20 percent, Dr. Blacker said.
That leads to the idea that if gene therapy floods the brain with APOE2, converting the brain milieu of a person with two APOE4 variants to one that resembles that of a person with one APOE4 and one APOE2, it could possibly slice Alzheimer’s risk in half.
Recruitment to try the technique has been slow, said Dr. Sam Gandy, professor of Alzheimer’s disease research at Mount Sinai in New York, who was one of the study investigators. Not everyone wants to sign up to have a virus carrying a gene injected into their brain.
But, he said, Alzheimer’s is so dreadful and people with two copies of APOE4 are at such risk that “desperate times call for desperate measures.”
The idea for the APOE2 gene therapy emerged 25 years ago, when both gene therapy and the discovery of the APOE variants were in their infancy. Three researchers who were then at Rockefeller University — Dr. Michael G. Kaplitt, now professor of neurological surgery at Weill Cornell Medicine, Dr. Gandy, and Dr. Paul Greengard — published an essay suggesting it.
But, Dr. Kaplitt said, the technologies at the time were not sufficient, and the researchers got busy with other projects.
The idea, he said, “languished.”
Now, with advances that make it feasible, researchers are able to use a harmless virus, A.A.V., to carry copies of the APOE2 gene to the brain. The virus and its gene cargo reach the brain after being injected directly into the spinal fluid.
Dr. Kaplitt, who is leading the trial, said that, for ethical reasons, he is not involved with Lexeo.
Dr. Robert C. Green, a medical geneticist at Harvard who has studied how people respond to knowing their APOE4 status, cautioned against leaping to conclusions on the basis of so little data from such a tiny study. However, he is not ready to dismiss it out of hand.
“It may be a Hail Mary treatment idea for Alzheimer’s disease,” he said. But “as a proof of concept,” he said, “I’m impressed.”
